Difference between revisions of "Lithium Ion Batteries"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
{{Energy Storage Technology Simple | {{Energy Storage Technology Simple | ||
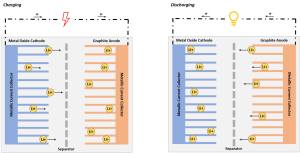
|imgdesc=An image showing the general chemical structure of a lithium ion cell | |imgdesc=An image showing the general chemical structure of a lithium ion cell. Image Source: The Economist (2002)<ref>The Economist: [https://www.economist.com/technology-quarterly/2002/06/22/hooked-on-lithium Hooked On Lithium (Jun. 22nd 2002)]</ref> | ||
}} | }} | ||
Revision as of 13:36, 20 December 2023
| Basic Technology Characteristics An image showing the general chemical structure of a lithium ion cell. Image Source: The Economist (2002)[1] | |
|---|---|
| How it Works: | Shuttle lithium ions (Li+) between cathode (+) and anode (-). Fully charged when Lithium ions are fully intercalated in the anode. |
| Benefits: |
|
| Challenges: |
|
| Technology Variations: | Lithium Iron Phosphate (LFP),
Nickel Manganese Cobalt (NMC), Nickel Cobalt Aluminum Oxide (NCA), Lithium Titanate Oxide (LTO) |
| Applications: | Diverse applications from minutes to hours duration and from small scale residential to transmission connected. |
| AC RTE Efficiency: | 80-92% |
| Cycle Life: | 3,000 - 10,000 cycles
10 - 20 years |
| Technology Readiness Level (TRL): | 9 - Deployed |
| Installed Capacity: | >10 GW |
- ↑ The Economist: Hooked On Lithium (Jun. 22nd 2002)